We offer various molecular biological, microbiological and biotechnological testing and development services. This can be studies or the implementation of a project. We accompany you from the idea of a new product or a new process to its market launch.
Our accredited testing laboratory (department for food testing) at the fzmb offers a wide range of testing services in the field of microbiological and chemical analyses.
Our Inter-Array division offers spotting services, e.g. for multi-parameter assays.
In the mylab system solutions division, you will find measuring devices for quality control, e.g. in the production of foodstuffs, as well as services and products in the fields of environmental analysis or laboratory and point-of-care diagnostics.
The veterinary clinic at our institute treats large and small animals in almost all medical areas. In addition to the small animal practice, our veterinary clinic focuses in particular on the medical care of horses.
Diagnostic systems

Development and production of microarrays
Microarrays consist of a large number of microscopic spots that can be precisely deposited on a solid surface by high-precision dispensers. For example, more than 700 spots can be applied in a single well of a 96-well microtiter plate.
Customized development
In the business unit INTER-ARRAY at the fzmb we accompany you through all development phases of microarrays from feasibility studies to pilot production. After successful development, we are available to you as a contract manufacturing partner with our ISO-certified production capacities.
Microarray quality control
The focus is on the high quality of the microarray products. For production, the dispensing systems operate in a climate-controlled environment with inline QC software, ensuring quality control of all arrays produced.
Information
For more information, visit our website at www.inter-array.com.
Contact
Katrin Frankenfeld
Head of Biotechnology Department
phone +49 3603 – 833 141
Development and production of multiparameter tests
Protein and antibody microarray technology offers enormous potential for a wide range of diagnostic applications. Miniaturized, parallelized immunoassays as well as DNA-based microarrays are ideal for generating maximum diagnostically relevant information with small sample and reagent volumes. A microarray thus saves a lot of time, valuable samples and expensive raw material.
Application in human and veterinary medicine
The use of microarrays in human and veterinary diagnostics is of interest for all applications where multiple analytes can be determined from a single sample.
Typical examples of multiparameter analysis are allergy and food intolerance tests, the diagnosis of autoimmune, metabolic and infectious diseases, and cancer diagnostics. The technology offers excellent prospects for new ways of individualized diagnostics and personalized medicine.
Multi-parameter analysis in different formats
In the business area INTER-ARRAY at the fzmb we carry out new developments of various multiparameter tests. Standard detection formats such as ELISA, lateral flow strips, and microfluidic assays can be used, as well as custom platforms.
Our team offers flexible support for feasibility, validation as well as production projects for various microarray based assays. In addition to completely new development, the transfer of existing tests to the microarray platform is also possible.
Information
For more information, visit our website at www.inter-array.com.
Contact
Katrin Frankenfeld
Head of Biotechnology Department
phone +49 3603 – 833 141
High precision dispensing
In order to apply very small volumes of capture molecules (from picoliters to microliters) to any surface with high precision, a lot of experience is required in addition to very good dispensing systems. Our INTER-ARRAY business unit at fzmb is equipped with a wide range of state-of-the-art microdispensers and has many years of specialist know-how.
From design to data analysis: Exclusive printing offers
With our established technologies for high-precision dispensing and our years of experience from design to data analysis, we create an exclusive printing offering in the life science sector. We offer this all-round service at an attractive price/performance ratio. The focus is on high quality and short production times.
Spotting of various substances
Substances that can be spotted include antibodies, other proteins and peptides, oligonucleotides, carbohydrates, cells and cell lysates, particles and chemical compounds, and special customer-supplied molecules. Substrates to be coated include glass and polymer slides, microtiter plates and microcavities, membranes and microfluidic chips, wafers, biosensors, and customized surfaces.
Information
For more information, visit our website at www.inter-array.com.
Contact
Katrin Frankenfeld
Head of Biotechnology Department
phone +49 3603 – 833 141
Immunoassay development
The lateral flow assay (LFA) is an immunological strip test. It combines the principle of thin-layer chromatography with antibody-based detection (colorimetric, enzymatic or fluorescence). An LFA is the basis for cost-effective, highly sensitive and reliable detection of a wide range of analytes.
As a point-of-care test (POCT), lateral flow assays find multiple applications e.g. in immunodiagnostics or in the analysis of environmental and food samples.
The ELISA(enzyme-linked immunosorbent assay) is used for the qualitative and/or quantitative detection of an analyte (antigen) in a sample. In addition, many samples can be analyzed in parallel and automated in a laboratory environment.
We develop LFAs and ELISAs from feasibility to small-scale production or preparation for large-scale production. These immunoassays can be used to diagnostically detect a wide variety of analytes in complex sample matrices. Highly specific and affine monoclonal and polyclonal antibodies are required for reliable selective binding of the analyte in a liquid phase or tissue sample. Their production and application-related characterization is one of our core competencies.
Reliable detection of biological structures with high sensitivity
Imunoassays can detect the analytes of interest down to the nanogram range, in some cases even the picogram range. Qualitative and quantitative detection is possible from a wide variety of biological samples with complex compositions without pretreatment.
At fzmb, rapid highly specific test systems with colorimetric, fluorescence or chemiluminescence-based readout for any parameter are developed.
Performance overview
- Development of ELISAs for the qualitative detection of micro- and macromolecules (formats: indirect ELISA, sandwich ELISA).
- Development of ELISAs for qualitative detection of analytes (format: sandwich ELISA).
- Development of lateral flow assays (LFA) for qualitative/quantitative detection of biomarkers.
- Optimization of assay components and steps for the benefit of sensitivity and assay speed
- Validation of the developed ELISA/ LFA
Contact
Highly sensitive 4in1/ 6in1 antigen rapid tests
The lateral flow assay (LFA) is an immunological strip test. It combines the principle of thin-layer chromatography with antibody-based detection.
At fzmb, we develop LFAs from feasibility to small-scale production or preparation for large-scale production.
More information from a quick test
Is it covid-19, RSV, or influenza (A or B)? If more than one question arises the answer should be sufficient, too We have therefore developed a new platform for lateral flow assays. On this new platform, up to 6 different tests can be integrated in one run (6in1 combinatied rapid tests).
Use of special nanoparticles
We use special fluorescent nanoparticles as a basis, which we conjugate with the corresponding antibodies. Thus, we achieve not only multiplexing (several tests combined). The sensitivity (detection limit of the respective analyte) is improved by up to 10 times.
The readout of the combination tests is done digitally with an appropriate readout device. Two variants are available for this purpose: For laboratory applications, commercially available devices (lateral flow fluorescence readers) can be used. In cooperation, we also offer solutions ready for series production for home use.
PDF Highly sensitive combination rapid tests
Contact
Specific antibodies
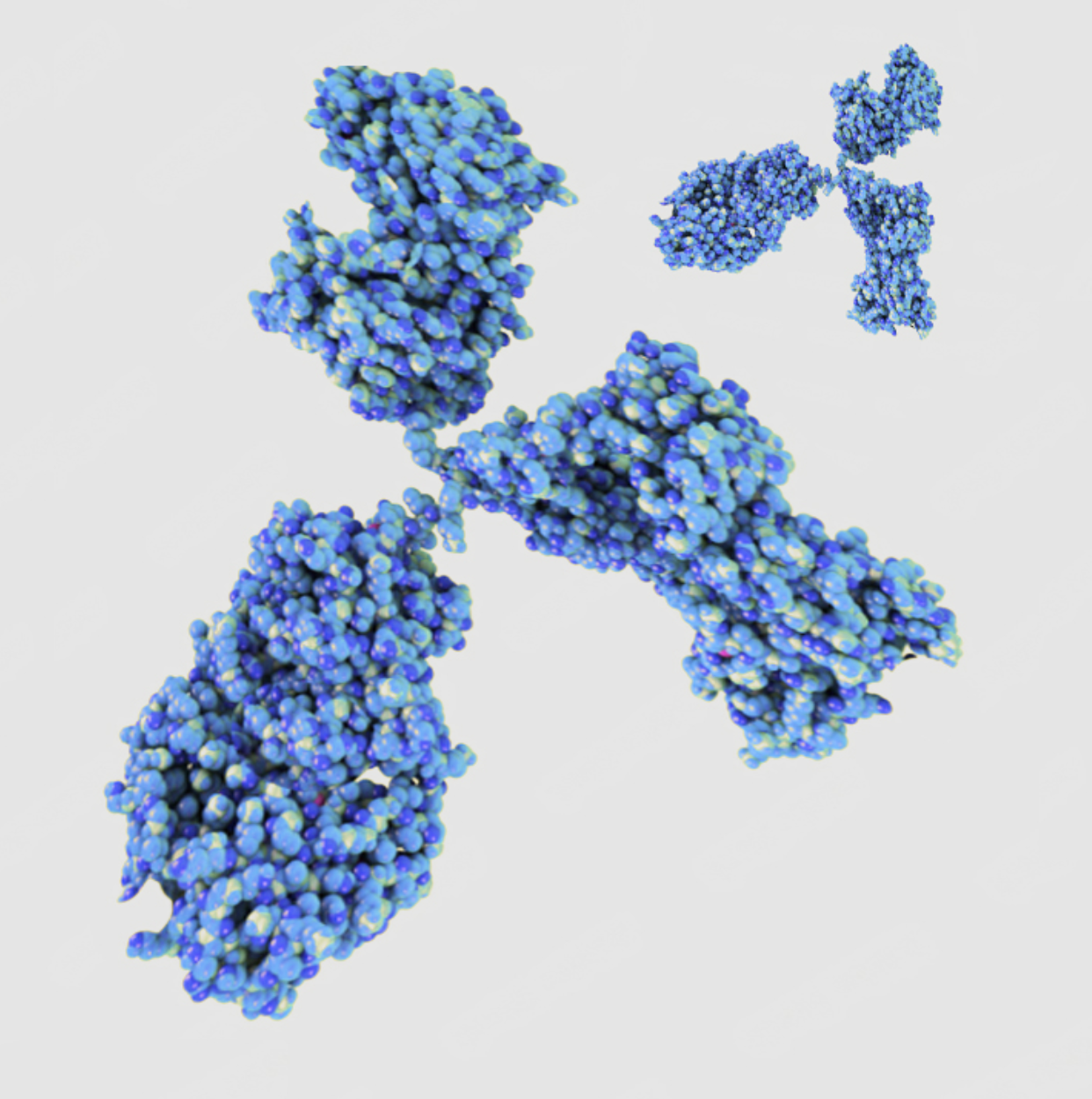
Production of monoclonal antibodies
Monoclonal antibodies can be generated for almost any antigen or epitope. These are the basis for various immunological-based detection methods, such as diagnostic ELISA tests. Unlike polyclonal antibodies, monoclonal antibodies are only produced by one cell clone (monoclonal) and therefore bind highly specifically to the epitope of the desired target structure.
Application-related characterization of monoclonal antibodies
We use the mouse host organism to develop monoclonal antibodies. The antibodies are generated using hybridoma technology, i.e. the fusion of spleen cells and myeloma cells.
For rapid downstream development, we immediately offer antibody characterization and matched-pair analysis. This identifies the pairings of detector and capture antibodies that are best suited to your desired test system.
Also production of antigens
In addition to the production of antibodies, we also produce antigens. The antigens can be recombinant and native proteins, peptides or microorganisms and can be used, for example, to develop specific antibodies.
The most important advantages at a glance
- No batch variance
- High specificity and selectivity
- Continuous secretion through hybridoma cell culture
Performance overview
- Production/procurement of the antigen
- Immunization of laboratory animals (mouse)
- Checking the immune response (titration of sera)
- Generation of antibody-producing clones using hybridoma technology
- Screening of clones and specificity testing
- Characterization of the antibodies (e.g. isotyping, expression performance, affinity to the antigen)
- Establishment of the selected clones as a cell line including cryopreservation in liquid nitrogen
- Antibody production and isolation by affinity chromatography from cell culture supernatant
- Integration in immunoassays of different formats (ELISA, LFA, IFAT)
Contact
Overview of monoclonal antibody-producing cell lines at the fzmb
monoclonal antibodies directed against
- Alpha1 proteinase inhibitor (dog) against canies alpha1PI
- Archaea ssp. (e.g. Methanosarcinae) against M. mazei Gs14, M.mazei DSM2053, M. flavesc.
- BBI against Bowman-Birk Inhbitor
- Clostridium botulinum neurotoxin C against BoNT-C
- Coxiella burnetii against Coxiella burnetii Phase I or II
- DON against the mycotoxin deoxynivalenol
- Fluorescent dyes and quenchers against FITC, Dyeomics 405, Dyomics 495, Dyeomics Quencher Q1, Dyeomics Quencher Q3
- Factor X against human factor X
- Factor Xa against human faxctor Xa
- American foulbrood(AFB)
- feline trypsinogen g
- Heparin Binding Protein against human heparin
- HIS-Tag against poly6-HIS-Tag
- Legionella pneumophila against various serogroups from SG1 to SG14
- Lunasin
- MAP against Mycobacterium avium paratuberculosis (inactivated cells)
- eMMP9 against equine matrix metallo proteinase 9
- NDV against Newcastle Disease Virus
- fNTproBNP (feline natriuretic brain peptide)
- cNTproBNP (canine Natriuretic Brain Peptide)
- PMSG against equine pregnant mare serum gonadotropin
- S. aureus LukF-P83 against Panton valentin leukocidin LukF-P83
- S. aureus PVL against Panton valentin leukocidin LukF-PV
- Salmonella ssp. against Salmonella enterititis
Contact
Production of recombinant antibodies (horse)
In human medicine, so-called chimeric (about 75 % human) and humanized antibodies have been used for more than 20 years. There are now a large number of approved therapeutic antibodies for human use. As a company with strong ties to veterinary medicine, our mission is to develop novel immunological therapies for companion animals.
Specific antibodies for therapy
We are developing recombinant equine-specific antibodies for subsequent therapeutic use in horses. The current focus is the development of an antibody against matrix metalloproteinase 9 (MMP-9). This antibody is intended to serve as a specific therapeutic agent, for example, for the targeted treatment of arthritis, wound healing disorders and equine sarcoid. But also in other diseases in which an overexpression of matrix metalloproteinase 9 (MMP-9) is causally involved, a new therapeutic option is to be created. In this way, pathological processes that arise due to the tissue-degrading properties of MMP-9 can be specifically stopped at the site of their development.
recombinant equine immunoglobulins
Molecular genetic methods have been developed for the production of equine recombinant antibodies in cell culture. Furthermore, these techniques can be used to produce other equine immunoglobulins, for example, as passive immunotherapeutics against equine infectious diseases. Based on the choice of subtype specificity and the regulatory Fc region, beyond the desired antigen specificity, the potential as a modulator can be used to strengthen the body’s immune system, e.g. against an existing cancer.
Go with us conversation about your concern and issue. Our ambition is to work with you to lay the foundations for innovative and specific immunotherapy for the horse.
If we have aroused your interest, please feel free to contact us.
Contact
Dr. Ina-Gabriele Richter
phone +49 3603 – 833 177
Production of polyclonal antibodies
We produce artificial human immune sera by means of in-vitro immunization.
Contact
Dr. Ina-Gabriele Richter
phone +49 3603 – 833 177
Matched Pair Antibodies
Whether a chosen matched-pair antibody works well in a planned application depends on many factors and cannot be predicted.
We therefore not only offer the development of specific monoclonal antibodies (including the identification of matched pairs) for e.g. Immunoassay developments on.
For best performance of a Matched Antibody Pair, we test the performance of these antibody pairs in the desired application. This early testing of antibodies in the target application (ELISA, LFA with immunogold or fluorescent nanoparticles, IFAT) enables targeted antibody selection or development and thus allows the best possible performance.
Performance overview
- Development of monoclonal antibodies against desired target structures
- Antibody characterization (isotyping, expression)
- Matched pair analysis in target format (sandwich ELISA, lateral flow assay LFA):
- Coupling to structures for colorimetric or fluorimetric detection (biotin, HRP, AP, fluorescent dyes).
- Immobilization of the catcher candidates
- Detectors analysis
- Testing of possible combinations
- Buffer optimization
Contact
Analysis of food, drinking water and cosmetics

Laboratory for food testing
The testing laboratory in the food testing department at the fzmb has been an independent service provider for food safety and consumer protection since 1994. The laboratory is accredited by the German Accreditation Body (DAkkS) in accordance with DIN EN ISO 17025.
Which products do we analyze?
- Meat and meat products
- Milk and dairy products
- Ice cream
- Bakery products
- Cereals and cereal products
- Delicatessen salads
- Fish
- ready-to-eat meals
- Drinking water
- other products on request
What services do we offer?
Food Microbiology
- Testing in accordance with legal requirements under Regulation (EC) No. 2073/2005 or in accordance with requirements of the German Society for Hygiene and Microbiology (DGHM) for products at retail level
- Surface germ content on carcasses
- Pathogen monitoring by means of PCR
- Salmonella antibodies in meat juice and serum
Industrial hygiene
- Control of cleaning / disinfection
- Control of personnel hygiene
- Airborne germ content (incl. sampling)
- Pathogen monitoring
Food Chemistry
- Mycotoxins (ochratoxin A)
- Main ingredients
- Nutritional values
- Nitrite/ Nitrate
chemical-physical
- aw value
- pH value
- Protective gas measurement
Drinking water
- Sampling
- Microbiology according to drinking water ordinance
other services
- Sample collection in modern refrigerated vehicle
- Checking the declaration for legal conformity
- Sensory testing
- Hygiene training
- Plant inspections/ hygiene audits
- Cross-check expert opinion
- Consulting and assistance with food law issues and the solution
concrete problems in the following areas:
- Development, production, storage, labeling and marketing of food products.
PDF List of test methods in the food testing department
Contact
Steffi Leuschke
State-certified food chemist
phone +49 3603 – 833 173
Microbiological and chemical analysis of food
As As an accredited testing laboratory, we carry out microbiological, chemical and chemical-physical testing of foodstuffs, including product-specific testing:
- Pathogen
- Spoilage agent
- Compliance with legal requirements (e.g. VO 2073/2005, Zusatzstoff-Zulassungsverordnung, Käseverordnung)
- Compliance with microbiological guideline and warning values of the German Society for Hygiene and Microbiology (DGHM)
- aw value and pH value
- Determination of food ingredients
- Compliance with specifications of the guiding principles of the German Food Book
- Nutritional value determination “BIG 7
- Determination of ochratoxin A in cereals and cereal products by HPLC.
- Detection of antibodies against Salmonella serovars of groups B, C, D and E in meat juice and blood products by ELISA, entry of results in the Qualiproof database.
- Checking of markings for legal conformity
Contact
Steffi Leuschke
State-certified food chemist
phone +49 3603 – 833 173
Microbiological testing of cosmetics
Microbiological product safety is one of the most important factors in the safety evaluation of cosmetic products. Our analytical laboratory tests the microbiological quality of cosmetic products as well as the raw materials used. Within the scope of operational hygiene monitoring, we offer manufacturers of cosmetics various tests of process water or production water, of room air and surfaces as well as of packaging materials.
The spectrum of our laboratory includes the following analysis methods:
- Preservation load test
(Evaluation of the preservation of cosmetic formu-.
ren)
- Testing for microbial contamination
- Detection of specific microorganisms
- Checking the cleaning and disinfection of surfaces and consumer goods
- Checking the indoor air quality
PDF Test laboratory for cosmetics
Contact
Steffi Leuschke
State-certified food chemist
phone +49 3603 – 833 173
Examination of air, objects, environmental samples
- Checking the cleaning and disinfection of surfaces and consumer goods
- Checking the indoor air quality
- Verification of personnel hygiene
Contact
Steffi Leuschke
State-certified food chemist
phone +49 3603 – 833 173
Drinking water testing
Drinking water is a foodstuff and is therefore subject to strict regulations. The testing of drinking water in our laboratory is carried out in accordance with the Ordinance on the Quality of Water for Human Consumption (Drinking Water Ordinance – TrinkwV 2001).
As an accredited testing laboratory (approved testing center according to § 40 para. 1 of the Drinking Water Ordinance), we are happy to check whether the quality of your drinking water complies with Directive 98/83 EC.
- Checking the microbiological drinking water quality (colony count at 22°C and 36°C, coliform germs, Escherichia coli, enterococci)
Additionally we offer:
- Determination of Pseudomonas aeruginosa
- Determination of Clostridium perfringens
- Testing drinking water installations for Legionella spp.
Note: If the testing of your drinking water must meet regulatory requirements, sampling by our accredited personnel is required.
PDF Test laboratory drinking water
Contact
Steffi Leuschke
State-certified food chemist
phone +49 3603 – 833 173
Biological recyclables
Inactivation of bacteria and viruses
The use of inactivated microorganisms as control material or for immunization for antibody production can minimize the risk of infection and contamination. For this purpose, the corresponding microorganisms are inactivated by a special UV treatment.
UV inactivation of microorganisms
UV-inactivated bacteria and viruses are particularly well suited as microbiological control material for scientific or medical purposes. The high-energy UV light damages the DNA, causing it to be inactivated. However, the macromolecular outer surface remains intact. UV-inactivated bacteria are therefore extremely useful for the production of antibodies against surface antigens.
If inactivated bacteria are used as reference material to vital bacteria, comparable results are obtained. UV-inactivated microorganisms are also ideal for evaluating molecular tests as standardized, independent quality controls.
Inactivation according to your specifications
We offer UV-inactivated microorganisms as whole cell preparations (e.g. inactivated bacteria such as Legionella or Streptococcus or other species according to BSL1 and BSL2). You receive the UV-inactivated MOs in suspension with a set cell count and determine the desired buffers and additives.
Please feel free to contact us so that we can discuss your individual requirements.
Literature
Mertens-Scholz, K., Moawad, A. A., Liebler-Tenorio, E. M., Helming, A., Andrack, J., Miethe, P., Neubauer, H., Pletz, M. W., Richter, I. G., (2024). Ultraviolet C inactivation of Coxiella burnetii for production of a structurally preserved whole cell vaccine antigen. BMC Microbiol 24(1): 118.
Information as PDF
Contact
Dr. Ina-Gabriele Richter

phone +49 3603 – 833 177
Inactivation of bacteria and viruses
The use of inactivated microorganisms as control material or for immunization for antibody production can minimize the risk of infection and contamination. For this purpose, the corresponding microorganisms are inactivated by thermal, chemical or UV treatment.
Customized inactivation
We offer inactivated microorganisms as whole cell preparations (e.g. inactivated bacteria such as Legionella or Streptococcus) as standardized, independent quality controls for the evaluation of molecular tests. We can also carry out customized inactivation, e.g. of bacteria, according to your specifications. You determine the desired concentrations, buffers and additives.
Please feel free to contact us so that we can discuss your individual request.
Thermal inactivation of bacteria and viruses
Unlike UV irradiation, our thermal process can also inactivate large quantities of microorganisms in a short time. The specific conditions for inactivation depend on the type of organism.
For example, inactivated bacteria are particularly suitable for generating antibodies against surface antigens. If inactivated bacteria are used as reference material to vital bacteria, comparable results are obtained.
Information as PDF
Contact
Dr. Ina-Gabriele Richter

phone +49 3603 – 833 177
BioCellulose (Made in Germany)
Ultra thin & ultra light
Natural skin feeling
Our BioCellulose is a pure natural material. It is significantly purer than plant cellulose and consists of a stable mesh of ultra-thin fibers that adapt perfectly to any skin contour. The masks are particularly light and transparent and therefore interesting even for those who have not used fleece masks so far.
BioCellulose as active ingredient carrier
In its meshwork of millions of fine fibers, our BioCellulose stores cosmetic active ingredient solutions for the treatment and care of the skin. Due to the optimal adaptation to the skin surface, the active ingredient molecules are specifically delivered to the skin and very good cosmetic results are achieved.
Application
Easy application in the beauty salon or at home
The BioCellulose masks are incredibly light and thin. As a result, they lie almost invisibly on the face. The application of the masks is very simple. The carrier material used allows easy application of the BioCellulose to the skin. The two mask parts allow adjustment to any face size. Due to the extremely good adhesion of the BioCellulose to the surface of the face, the client has a high degree of freedom of movement during the treatment.
Ideal combination with apparative treatment methods
BioCellulose masks provide a unique basis for combination with apparative treatment methods. The material of the masks allows, in addition to an optimal release of active ingredients, a perfect transfer of the physical effects to positively influence regeneration processes of the skin and stimulate metabolic processes.
Production
Development, production and packaging
We act as a contract manufacturer for the production of cosmetic masks according to customer requirements. Our BioCellulose is biotechnologically extracted from glucose in Germany and processed into masks. We impregnate the masks with our customers’ active ingredients or provide a product formulation. We are happy to advise on the choice of active ingredient.
Research and development
We implement the ideas that our customers bring to us. The production of samples is part of our service offer. With our own perfectly equipped laboratories, our competent chemists, biologists and laboratory technicians are able to offer comprehensive research and development services.
Quality
We have been producing BioCellulose at the highest level for 20 years. Our experienced team works to produce high quality products to exacting standards. The GMP-compliant production is certified according to DIN ISO 9001. The product is dermatologically tested and is manufactured entirely in Germany. We guarantee fast order processing.
Contact
Dr. Ina-Gabriele Richter
phone +49 3603 – 833 177
Data Science & Optical Analytics

Hyperspectral Imaging (HSI)
Quality control of production flows
An important field of application of hyperspectral imaging is quality control, e.g. in food production. The fast and continuous data acquisition and the evaluation in real time create an excellent basis for inline controls of production flows. You can find examples of this here.
Hyperspectral Imaging (HSI)
Every surface and every material has its own spectral signature. This characteristic signature can be recorded with a hyperspectral camera. Algorithms evaluate the signatures and assign them to qualitative and quantitative properties of the measurement objects.
Functionality HSI
Like a scanner, many hyperspectral cameras capture their images line by line. For this purpose, the sample to be examined is moved under the camera. A characteristic spectrum of the measured object within a defined wavelength range is detected in each image point.
Data evaluation in real time
Modern evaluation methods allow very detailed information about the measured object to be generated in real time from the highly complex measurement data. For example, the quantitative chemical composition of food can be determined. The method also enables the detection of foreign objects as well as the detection of quality deviations or contamination of individual objects in the production flow. Continuous data acquisition and evaluation enables complete coverage of the entire product flow. The often time-consuming and unrepresentative taking of random samples is no longer necessary.
HSI integration into your operation
With our HSI modules, objects from a few micrometers up to 60 cm can be analyzed in the wavelength range of 400 – 2500 nm. Our laboratory first performs preliminary examinations of your measurement objects and material samples. Based on the results, we configure a customized in-process system for you, integrating special AI-based evaluation methods.
PDF Hyperspectral Imaging
Contact
Florian Meuche
Department Manager Equipment Development
phone +49 3603 – 833 194
NIR spectroscopy
Organic samples absorb light in the near-infrared (NIR) wavelength range of 800-2500nm. Substances contained in the sample, such as proteins, fats, sugar and water components, generate a characteristic NIR spectrum. The analysis can be performed both qualitatively and quantitatively. Physical parameters such as particle size or pH also influence the shape of the NIR spectrum and can be determined.
NIR spectroscopy thus offers fast, non-contact and precise measurement results, e.g. in the food and feed industry. Compared to chemical analyses, NIR measurements are much faster (>60 sec), require no special sample preparation and can analyze a large number of parameters in parallel.
How NIR spectroscopy works
The special excitation of the sample excites characteristic molecular vibrations. The vibrational states within the sample are reflected in the detected NIR spectrum. An analysis of the shape and position of the measured spectrum makes it possible to calculate the chemical composition/physical parameters of the sample.
Integration of NIR spectroscopy into your operation
First, your samples are measured in our spectroscopy laboratory. At the same time, your desired target parameters are determined in our accredited analysis laboratory.
Then the NIR spectrometer system mylab-NIR Analyzer and evaluation model is optimized for your measurement task. Integration into your production process is individually tailored to your requirements.
Contact
Florian Meuche
Department Manager Equipment Development
phone +49 3603 – 833 194
Ion Mobility Spectrometry (IMS)
Rapid analysis of volatile substances
Ion mobility spectrometry (IMS) analyzes volatile compounds for their chemical composition. It is characterized by low detection limits (lower ppb range) and short measuring times (approx. 20 sec).
Functionality IMS
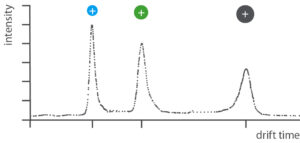
Defined heating of the sample brings the chemical substances into the gas phase. The now gaseous substances are first ionized in the IMS. Driven by an electric field, the ions move through a gas-filled tube (drift tube).
In the drift tube, the ions encounter gas molecules (e.g. air). The larger the ions in a sample, the more frequently they collide with the gas molecules and the longer they take to pass through the drift tube.
After passing through the drift tube, a detector determines the drift time. The longer the drift time, the greater the molecular weight of an ion.
Examination of complex gas samples
For the analysis of complex gaseous samples, sample pre-separation by gas chromatography (GC) is a suitable method. The instrument configuration established at fzmb allows the analysis of light to medium volatile compounds up to a boiling point of 120 C°.
The range of substances that can be analyzed includes organic compounds such as hydrocarbons, aldehydes, ketones, alcohols, esters and amines.
Data evaluation
The analysis of the IMS data includes the detection of patterns in the measured spectra. This pattern recognition helps assign ions to specific compounds and identify unknown substances. By creating classification models, the precision of the analytics is improved.
Further data evaluation concerns the concentration determination of the analyzed compounds. For this purpose, the intensities of the ion peaks are measured and compared with calibration standards or internal standards. Data obtained in this way are used for regression models.
Further information
You can find out more about ion mobility spectrometry here.
Range of services
- Fingerprint analytics
Comparative analysis of two different samples
(e.g. healthy vs. sick, treated vs. untreated).
Substratification for the identification of differentially expressed substances
- Identification of unknown substances (e.g. by GC-MS)
- Quantitative analysis of known (or previously characterized) substances
IMS integration into your operation
Areas of application at fzmb:
- Microbiology and diagnostics of infections
- Reproductive medicine in farm animals
- Proof of medication for sport horses and food-producing animals
- Detection of ingredients in animal tissues
- Detection of impurities and additives in plastics
PDF Ion Mobility Spectrometry
Contact
Florian Meuche
Department Manager Equipment Development
phone +49 (0)3603 – 833 194
Development of optical measuring instruments: Feasibility studies and validations
Optical measuring instruments
The composition and surface properties of materials can be characterized by interactions between light and matter. Modern optical analyzers use physical effects such as absorption, reflection, transmission or the scattering of light to measure certain properties of a sample.
Smart development saves costs
For the development of efficient and cost-optimized optical analysis instruments, precise knowledge of the optical properties of the samples to be analyzed is essential. Based on this knowledge, optimally tailored technical solutions can be designed. This can reduce both development time and costs (for hardware, among other things).
State-of-the-art test laboratory and expertise
With our extensively equipped spectroscopy laboratory, our expertise in the field of optical metrology, modern simulation tools and innovative AI-based evaluation methods, we support you in the development of optimized optical measurement and analysis devices.
Our services
- Measurement of absorption, reflection, transmission and scattering in the wavelength range of 200 – 4000 nm (UV – MIR) for the identification of problem-relevant wavelengths
- Simulation of the position and performance of light sources for the selection of suitable components
- Simulations for the optimization of beam paths
- Simulation of required detector areas for photodiodes
- Consulting on the design of electronic circuits for signal acquisition and processing
Contact
Florian Meuche
Department Manager Equipment Development
phone +49 3603 – 833 194
Overview services
- Measurement of absorption, reflection and transmission in the wavelength range from 200 – 2,800 nm
- Scattered light measurements
- time-resolved fluorescence spectroscopy
- Near Infrared Spectroscopy
- Hyperspectral measurements in the wavelength range from 400 – 2,500 nm
- Development of chemometric models for NIR spectroscopy
- Development of AI-based evaluation methods for hyperspectral measurement data.
- Radiation optical simulations
- CAD modeling and 3D printing
Overview device equipment
- Stratasys OBJET 30 Prime Desktop 3D Printer
- Ocean Optics HR4000 CG UV-NIR Spectrometer
- BRUKER MPA – FT- NIR Spectrometer
- Hamamatsu FT – IR Spectrometer C12606-02
- ARCoptix FT- MIR Rocket 6.0
- Horiba Fluolog FL3-22
- Headwall Photonics VNIR Hyperspectral Imaging System
- SpecIm SP SP-sCMOS-CL50-v10E VIS-NIR Hyperspectral Imaging System
- SpecIm SP-SWIR-CL-400-N25E SWIR Hyperspectral Imaging System
Services of the veterinary clinic

The medical services of the equine clinic can be found on the website of the veterinary clinic Bad Langensalza.
The services of the small animal practice can be found on the website of the veterinary clinic Bad Langensalza here.